
There are subscripts, which are part of the chemical formulas of the reactants and products and there are coefficients that are placed in front of the formulas to indicate how many molecules of that substance is used or produced. Remember that if there's no coefficient in front of an element, it's assumed that the coefficient is 1.There are two types of numbers that appear in chemical equations. A balanced equation is a chemical reaction equation in which the total charge and the number of atoms for each element in the reaction are the same for both the. Apart from this, it is also capable of displaying the chemical structure and properties of a given substance, along with its chemical name and formula. Now the number of atoms in each element is the same on both sides of the equation, so the equation is balanced. The chemical equation calculator is a helpful online tool that can accurately balance a given chemical equation within seconds. The only files required are the program itself (duh) and Pic1. 2) When isopropanol (C3H8O) burns in oxygen, carbon dioxide, water, and heat are produced. 1) When dissolved beryllium chloride reacts with dissolved silver nitrate in water, aqueous beryllium nitrate and silver chloride powder are made. Split strong electrolytes into ions (the complete ionic equation). Chemical Equation Balancer Balances Chemicam Equations with up to 2 compounds in the product and reactant: basicperiodictable.zip: 2k: 10-04-17: Basic Periodic Table This is just like the Periodic app that comes with some calculators, except written in pure BASIC. Write and balance the following chemical equations. To balance this, add the coefficient 2 before H2 on the left side of the equation so there are 4 hydrogen atoms on each side, like 2H2 + O2 → 2H2O. Write the state (s, l, g, aq) for each substance. However, subscripts can't be changed and are always multiplied by the coefficient, which means there are now 4 hydrogen atoms on the right side of the equation and only 2 hydrogen atoms on the left side. For the equation H2 + O2 → H2O, you would add the coefficient 2 before H2O on the right side so that there are 2 oxygen atoms on each side of the equation, like H2 + O2 → 2H2O. Undoubtedly one of the most difficult subjects in school, chemistry is the science responsible for our. To balance the equation, you'll need to add coefficients to change the number of atoms on one side to match the other. Review by Mircea Dragomir on January 31, 2015.
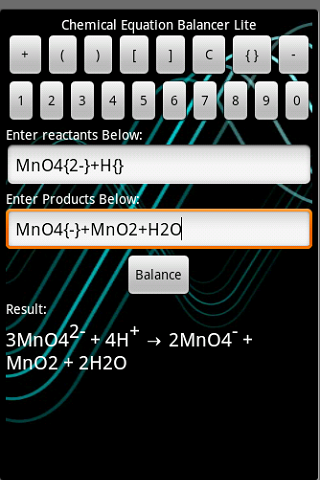
Since the number of atoms in each element isn't identical on both sides, the equation is not balanced. When balancing equations, the goal is to make sure that the same atoms, in both type and amount, are on both the reactant and product side of the equation.

There are 2 hydrogen atoms and 1 oxygen atom on the right, so you would write "H=2" and "O=1" under the right side. For the equation H2 + O2 → H2O, there are 2 hydrogen atoms being added to 2 oxygen atoms on the left, so you would write "H=2" and "O=2" under the left side. To balance a chemical equation chemists often guess the coefficients that would balance the. Export and share data instantly to excel. why it is necessary to use balanced equations in science. Perform stoichiometric calculations to find how much reagents and chemicals you need. Search Chemical Reactions from about 12000 reactions. Balance chemical reactions and equations. For example, your equation should look something like "H2 + O2 → H2O." Count the number of atoms in each element on each side of the equation and list them under that side. The description of Balance Chemical Equations - Equation Balancer App. Following are some equation input format examples: 1. This balancer can also help you check whether the equation is balanced or not, thus you may edit the equation and check it's balance. To balance a chemical equation, first write out your given formula with the reactants on the left of the arrow and the products on the right. This chemical equation balancer can help you to balance an unbalanced equation.


 0 kommentar(er)
0 kommentar(er)
